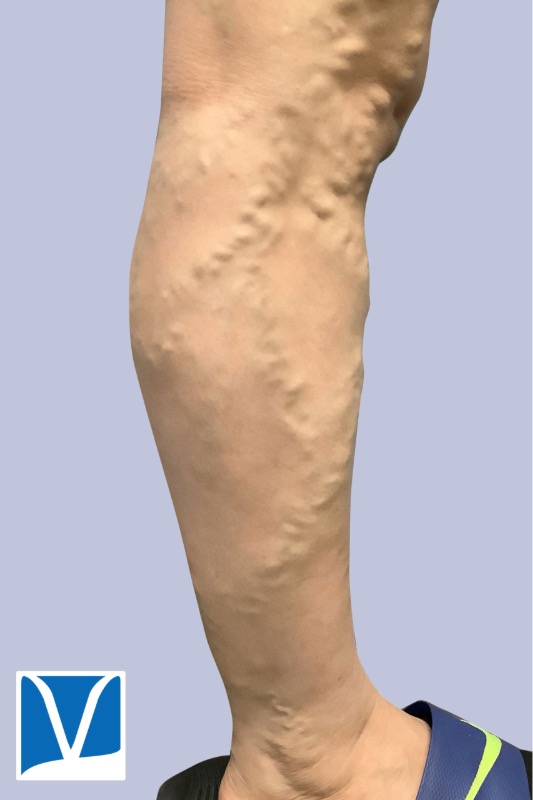
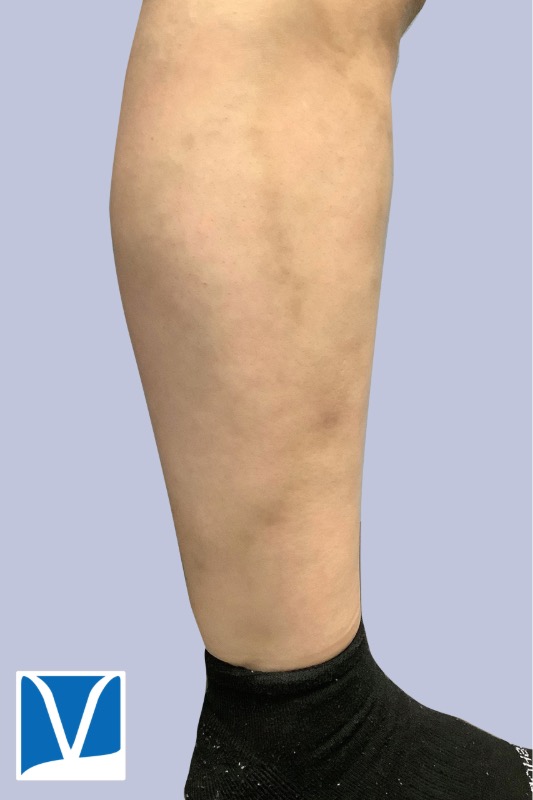
Varithena vein treatment for varicose veins is one of the most exciting developments in sclerotherapy in recent years. Sclerotherapy is commonly used approach to treat small, medium branch varicose veins, as well as spider veins. However the underlying source of these veins is often the greater saphenous vein (GSV) and small saphenous vein (SSV).
Over the years, it has been noted that sclerotherapy to treat the source sapheonous veins that cause most varicose veins is not as effective as treatments such as Closurefast RFA. Furthermore, sclerotherapy was not approved to treat the saphenous veins, nor was it covered by most insurance companies for this purpose.
Sclerotherapy is an injection of an FDA approved medication in either liquid form, or in some cases, as a foam. Common sclerotherapy agents in the united states include Polidocanol and Sotradecol. These agents were originally indicated to treat uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. There was not much evidence to support label indications for larger varicose veins (> 3 mm in diameter). Since the saphenous veins are generally far larger than 3 mm, these commonly available sclerotherapy agents were not optimal for the treatment of the saphenous veins that cause most varicose branch veins.
THESE PHOTOS ARE OF AN ACTUAL PATIENT OF OUR PRACTICE WHO HAS PROVIDED CONSENT TO DISPLAY THEIR PICTURES ONLINE. KEEP IN MIND THAT EACH PATIENT IS UNIQUE AND RESULTS MAY NOT OCCUR FOR ALL PATIENTS. PLEASE BOOK A CONSULTATION FOR MORE INFO ON HOW TREATMENTS MAY HELP YOU.
A stronger concentration and optimized foam preparation known to be required to treat the saphenous veins, but this was not commercially available in the United States. Recently, polidocanol endovenous microfoam (PEM) 1%, known as Varithena, was approved for use in the United States by the FDA. Varithena(polidocanol injectable foam) is a stronger concentrated commercially available foam indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. This is a specialty formulated foam sclerotherapy that retains its foam confirmation for a longer time, and therefore is more effective at closing the causative truncal veins such as the GSV and SSV.
Varithena is a drug/device combination product that produces engineered polidocanol endovenous microfoa. Varithena vein treatment is delivered from a canister through a syringe into the incompetent vein under ultrasound guidance. Varithena first displaces blood and then the polidocanol chemically ablates the inner lining of the vein wall, causing the vein to close.
In this procedure, a catheter is placed on the inside of the vein, and foam bubbles are injected under ultrasound guidance by the doctor, which causes the veins to spasm, and close off.
This approach is especially best suited for patients with complicated varicose veins, such as those patients who have had prior clots in the veins, making passage of a catheter up the vein more difficult.
The Varithena Vein Treatment Data
The use of Varithena as a treatment for varicose veins was evaluated in multiple clinical trials where it was shown to be safe and effective. In these studies Varithena vein treatment was shown to improve the symptoms of superficial venous incompetence and the appearance of visible varicosities. We participated in one of these market approval trials, known as VANISH II trial.
Based on the data from these studies, Varithena was approved by the FDA. Furthermore, after evaluation by CMS, Varithena now has insurance coverage for reimbursement when indicated by Medicare and some other insurance companies

Frequently Asked QuestionsVarithena
-
-
When can I travel after a Varithena procedure?
In most cases, patients are able to travel as soon as the day after a Varithena procedure. However, there are important considerations to take for long-term travel that may restrict leg movement.
-
How long does it take to recover from Varithena?
In most cases we ask patients to take it easy the first night, but back to usual activities the next day. There can be some tenderness along the treated veins and this can take a few weeks or longer to fully resolve. Most people find NSAIDs such as Ibuprofen or Aleve can help. Also, topical NSAIDs like Voltarin can help.
-
What should I do after Varithena?
The recovery is the same after most office-based vein procedures. The night of the procedure we generally advise the patient go home, put their leg up on some pillows, and take it easy. In most cases we ask they take it easy the first night, but back to usual activities the next day.
-
Is Varithena the same as sclerotherapy?
Varithena is a form of foam sclerotherapy. It is different than most forms of sclerotherapy in that it comes in foam form in a proprietary delivery canister.
-
Does Medicare cover Varithena?
Yes, Varithena is covered by Medicare for patients that meet the criteria for medical necessity. This is covered generally when it is for medical indications (such as to reduce swelling, ache, pain, skin changes etc) but not for purely cosmetic reasons.
-
Do Varithena injections hurt?
Varithena is an advanced proprietary form of sclerotherapy, a treatment that has been used for many years to treat varicose veins. Most patients do not complain that the procedure was painful. There is the sensation of needle sticks from the numbing medicine which is used before the catheter is placed. Sometimes there is a bit of burn when the medicine goes in, but this generally fades quickly. It requires far fewer pin pricks than other therapies such as Closurefast RFA.
-
Is Varithena safe?
Yes. This is an FDA approved foam medication that has been shown to be both safe and effective in clinical trials and community use. It is not without any risk, however. There is a risk of blood clots as there is with any leg procedure, as well as other risks that do not commonly happen. It is best to review the potential risks and benefits with your vein care provider before you have any procedures.
-
Learn more about Varithena Vein Treatment
At Inovia Vein Specialty Centers we are leading vein doctors who specialize in caring for patients with venous disorders such as varicose veins, deep venous thrombosis, and venous stasis ulcers. Founded in 2006, we have specialty vein clinics across the Pacific Northwest. Call to set up a consultation to learn more about your treatment options.